Abstract
Natural killer (NK) cells play a crucial role in recognizing and killing emerging tumor cells. Via an array of activating and inhibitory receptors, NK cells can target and kill abnormal cells, including tumor cells, without prior antigen sensitization. However, fully-established tumors employ numerous mechanisms to inactivate NK cells or hide from them, for example by shedding NK cell-activating ligands, like NK2GD (Chiossone et al., 2018). Here, we describe the engineering of nanoparticles (NPs) functionalized with tumor-recognizing and cytotoxic components of NK cells to address this problem and develop a nanoparticle-based treatment strategy simulating NK cell functionality.
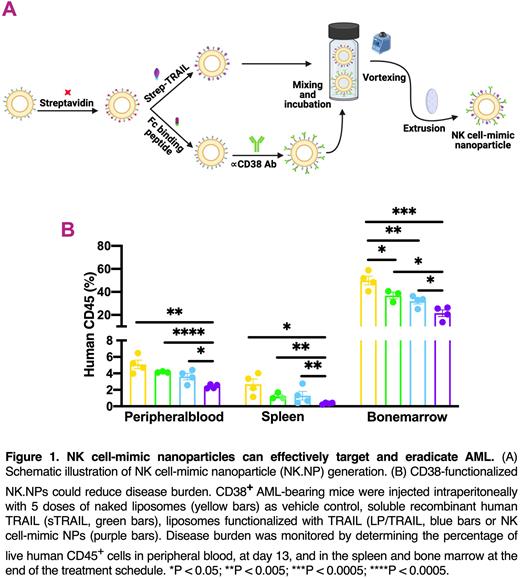
Liposomes were synthesized using the thin film hydration method followed by extrusion and functionalized them with the death ligand, TRAIL (tumor necrosis factor-related apoptosis-inducing ligand), immunoglobulin Fc-binding peptide (FCP, replicating the function of NK CD16 receptor) and the therapeutic antibody, anti-CD38 (daratumumab, Panel A) to target acute myeloid leukemia (AML). Transmission electron microscopy (TEM) and dynamic light scattering (DLS) were used to monitor morphology and size of liposomes. The potential of NK cell-mimic NPs (CD38-NK.NPs) to target and kill AML cells was evaluated on primary AML blasts and a disseminated AML xenograft for which the CD38-positive AML cell line, OCI-AML2 was engrafted into 8-9 week-old female NSG mice. Upon establishment of AML, the mice were injected intraperitoneally (i.p) with 5 doses of either naked liposomes (as vehicle control), soluble TRAIL protein (sTRAIL), LP/TRAIL or CD38-NK.NPs every two days. Tumor burden was monitored by determining the frequency of live human CD45+ cells in the peripheral blood, spleen and bone marrow with flow cytometry.
Liposome was chosen as the base of the NP due to its high biocompatibility, biodegradability, cell-like characteristics and capability to penetrate through vessel walls (Pattni, Chupin and Torchilin, 2015). TEM and DLS measurements showed the generated NK.NPs were monodisperse with an average diameter of 145 nm, a size enabling vessel transmigration and avoiding kidney filtration (Gaumet et al., 2008). Immunofluorescent labelling of TRAIL, FCP and antibody verified CD38-NK.NPs functionalization.
In vitro, both TRAIL-conjugated liposomes and CD38-NK.NPs were more effective in killing AML cell lines (ML-1, ML-2, Kasumi-1, and OCI-AML2) than sTRAIL, indicating that TRAIL-mediated cytotoxicity can be enhanced by presenting TRAIL in a conformation close to its membrane-bound structure. No significant difference could be detected between LP/TRAIL vs. CD38-NK.NP cytotoxicity in the in vitro assays. On the other hand, CD38-NK.NPs were more effective in killing primary AML blasts than non-targeted, NP-conjugated TRAIL (LP/TRAIL, 13±17% vs 4±15% killing, respectively). Moreover, CD38-NK.NPs showed considerably higher activity relative to the NK cell line, KHYG-1, against CD38-positive AML cell lines. Notably, CD38-NK.NPs showed no significant cytotoxic effect on non-malignant peripheral blood leukocytes.
In vivo, NK cell-mimic NPs were more efficient in targeting and eradicating CD38-positive AML cells that either sTRAIL or LP/TRAIL, shown by the reduced percentage of circulating hCD45+ AML cells in treated mice (Panel B). CD38-NK.NPs were also more efficient in inhibiting accumulation of AML cells in the bone and spleen than sTRAIL and LP/TRAIL (Panel B), confirming the ability of CD38-NK.NPs to actively target AML cells expressing the targeted biomarker. Of note, CD38-NK.NPs showed no detectable systemic toxicity or damage to kidneys, liver and pancreas in healthy NSG mice.
Overall, we have developed an NK cell-mimic nanoparticle able to actively target AML based on AML surface marker expression without any detectable systematic toxicity. Importantly, due to the modular built of the NK.NPs, they can be functionalized by any therapeutic tumor-targeting antibody via the immunoglobulin-binding peptide (FCP), thus enabling selective targeting tumor cells based on expression of tumor-specific markers and enabling personalized therapy.
Disclosures
Szegezdi:ONK Therapeutics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal